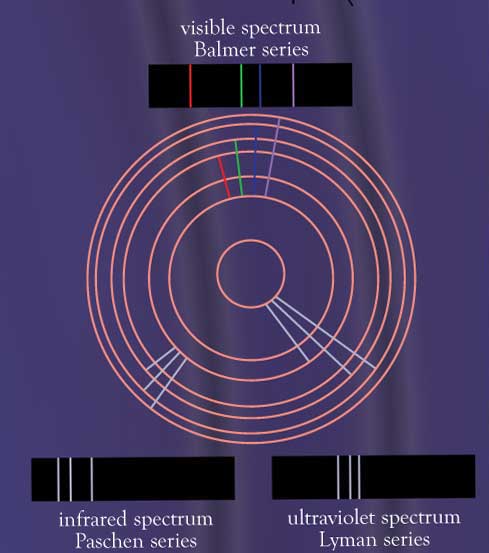
Observed atomic spectra result from electrons moving from a higher energy orbital to a lower energy orbital. The spectral pattern is distinctive for each element. As atoms absorb and release more energy, additional lines may appear, but the wavelength of each line will not change. This observation helped to develop the quantum model of atomic structure. It currently allows scientists to determine the makeup of unknown samples of matter and also predict the composition of distant stars.